Physiopathologie et Epidémiologie des Maladies Respiratoires
Présentation
Three main areas of investigation are currently being pursued in our team :
1) Study in vitro and in vivo of the role of Pseudomonas aeruginosa infections (an opportunistic pathogen in pulmonary nosocomial infection and in cystic fibrosis) in exacerbations of chronic lung diseases, including cystic fibrosis.
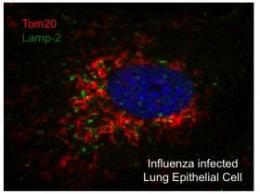
2) In vitro and in vivo study of the mechanisms through which Influenza virus, a pathogen responsible for lung infections in seasonal flu or pandemic episodes can favour bacterial super-infections and induce disease exacerbations in chronic lung diseases.
3) Investigation of the molecular mechanisms of action of nanoparticles in the lung, in isolation or in conjunction with lung pathogens (S. aureus, P.aeruginosa, Influenza virus…) and of their potential role as adjuvants.
The molecular processes, techniques, reagents, signalling pathways studied and used in this context include :
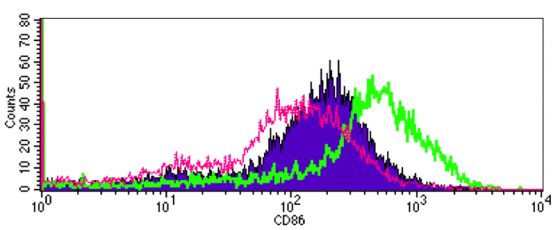
- Mechanisms of Pathogen Associate Molecular Pattern/PAMP receptors activation, phagocytic processes, signal transduction pathways ; role of epithelial type 2 serine proteases (TTSPs) during Influenza infection ; interactions between microbial virulence factors, innate immune receptors and epithelial channels (eg le CFTR) ; study of endogenous antimicrobial molécules ; use of recombinant adenovirus vectors in over-expression protocols ; in vivo rodent infection models ; RT-qPCR ; flow cytometry ; immuno-histochemistry ; proteomics.
Thèmes de recherche
Research themes :
At the surface of the epithelium and pulmonary alveoli, epithelial cells and innate immune cells (alveolar macrophages, NK cells, Innate lymphoid cells/ILCs..) interact, through the production of surfactant, regulatory cytokines and antimicrobial molecules, to ensure a non- inflammatory regulatory phenotype . After infective or toxic stimuli exposure, these cells participate in a network to organize pro-inflammatory responses and to engage adaptive immunity to contain the aggression and to insure the return to haemostasis. Our research group is mostly interested in the innate mechanisms put in place in this process and in the dysregulation of these control mechanisms, which could potentially explain the pathophysiology of some inflammatory disorders (chronic obstructive pulmonary diseases/COPD, cystic fibrosis…).
Equipes de recherche
Current team members :
- Pr J-M Sallenave, PU CE, Université Paris-Diderot
- Dr I. Garcia-Verdugo, MCU, Université Paris-Diderot
- Pr P. Montravers, PU-PH, Hôpital Bichat
- Pr A. Khalil, PU-PH, Hôpital Bichat
- Mrs B. Villeret, IE, Université Paris-Diderot
- Mrs R. Ghinnagow, Post-doctoral fellow, INSERM
- Mrs A. Picart, tech., INSERM
- Mr S. Kheir, PhD student, Université Paris-Diderot
- Mr D. Sanchez, M2 student, Université Paris-Diderot
Publications
Some recent publications (liste non exhaustive) :
- Villeret B, Dieu A, Straube M, Solhonne B, Miklavc P, Hamadi S, Le Borgne R, Mailleux A, Norel X, Aerts J, Diallo D, Rouzet F, Dietl P, Sallenave JM, Garcia-Verdugo I. Silver Nanoparticles Impair Retinoic Acid-Inducible Gene I Mediated Mitochondrial Anti-Viral Immunity by Blocking the Autophagic Flux in Lung Epithelial Cells. ACS Nano. 2018 Jan 22. doi: 10.1021/acsnano.7b06934.
- Saint-Criq V, Villeret B, Bastaert F, Kheir S, Hatton A, Cazes A, Xing Z, Sermet-Gaudelus I, Garcia-Verdugo I, Edelman A, Sallenave JM. Pseudomonas aeruginosa LasB protease impairs innate immunity in mice and humans by targeting a lung epithelial cystic fibrosis transmembrane regulator-IL-6-antimicrobial-repair pathway. Thorax. 2018;73:49-61
- Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, Serafini N, Puel A, Bustamante J, Surace L, Masse-Ranson G, David E, Strick-Marchand H, Le Bourhis L, Cocchi R, Topazio D, Graziano P, Muscarella LA, Rogge L, Norel X, Sallenave JM, Allez M, Graf T, Hendriks RW, Casanova JL, Amit I, Yssel H, Di Santo JP. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell. 2017 ;168:1086-1100.e10.
- Roussilhon C, Bang G, Bastaert F, Solhonne B, Garcia-Verdugo I, Peronet R, Druilhe P, Sakuntabhai A, Mecheri S, Sallenave JM. The antimicrobial molecule trappin-2/elafin has anti-parasitic properties and is protective in vivo in a murine model of cerebral malaria. Sci Rep. 2017;7:42243.
- Delaval M, Boland S, Solhonne B, Nicola MA, Mornet S, Baeza-Squiban A, Sallenave JM, Garcia-Verdugo I. Acute exposure to silica nanoparticles enhances mortality and increases lung permeability in a mouse model of Pseudomonas aeruginosa pneumonia. Part Fibre Toxicol. 2015 Jan 21;12:1. doi: 10.1186/s12989-014-0078-9.
- Brown TI, Collie DS, Shaw DJ, Rzechorzek NM, Sallenave JM. -Sheep lung segmental delivery strategy demonstrates adenovirus priming of local lung responses to bacterial LPS and the role of elafin as a response modulator. PLoS One. 2014;9:e107590
- Le Gars M, Descamps D, Roussel D, Saussereau E, Guillot L, Ruffin M, Tabary O, Hong SS, Boulanger P, Paulais M, Malleret L, Belaaouaj A, Edelman A, Huerre M, Chignard M, Sallenave JM. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med. 2013;187:170-9.
- Drannik AG, Nag K, Sallenave JM, Rosenthal KL. Antiviral activity of trappin-2 and elafin in vitro and in vivo against genital herpes. J Virol. 2013;87:7526-38.
- Descamps D, Le Gars M, Balloy V, Barbier D, Maschalidi S, Tohme M, Chignard M, Ramphal R, Manoury B, Sallenave JM. Toll-like receptor 5 (TLR5), IL-1β secretion, and asparagine endopeptidase are critical factors for alveolar macrophage phagocytosis and bacterial killing. Proc Natl Acad Sci U S A. 2012;109:1619-24.
- Garcia-Verdugo I, BenMohamed F, Tattermusch S, Leduc D, Charpigny G, Chignard M, Ollero M, Touqui L. A A role for 12R-lipoxygenase in MUC5AC expression by respiratory epithelial cells. Eur Respir J. 2012 ;40:714-23
- Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O'Neal WK, Sallenave JM, Pickles RJ, Boucher RC. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci U S A. 2012;109:16528-33
- Motta JP, Magne L, Descamps D, Rolland C, Squarzoni-Dale C, Rousset P, Martin L, Cenac N, Balloy V, Huerre M, Jenne D, Wartelle J, Belaaouaj A, Mas E, Vinel JP, Alric L, Chignard M, Vergnolle N, Sallenave J-M. Modifying the Protease, Antiprotease Pattern by Elafin Overexpression Protects Mice From Colitis. Gastroenterology. 2011; 140:1272-82
Enseignements
Teaching : click on Master Infectiologie, Master Inflammation, Université Paris Diderot.
Autres contacts
Pr Jean-Michel Sallenave
Tel : +33157277802
Fax : +33157277551
E-mail : jean-michel.sallenave@inserm.fr
Fiche Aviesan ITMO 'PMN' : https://pmn.aviesan.fr/index.php?pagendx=295&team=279 (internal link)